– Importance, Uptake, and Consequences
The ocean is currently absorbing more CO₂ than it naturally should. Between 1994 and 2007, the world’s oceans absorbed about 31 percent of all human-caused CO₂ emissions. Since the Industrial Revolution, the oceans have taken up about one-third of anthropogenic CO₂ each year.
The overload is evident in:
Ocean acidification due to massive CO₂ uptake
Decreasing absorption capacity as temperatures rise
Regional reversals, where some marine areas already emit more CO₂ than they absorb.
The Ocean as the Largest Carbon Reservoir on Earth
The world’s oceans act as the largest active carbon reservoir on our planet. With about 38,000 gigatons of stored carbon, they surpass the terrestrial biosphere by 16 times and the preindustrial atmosphere by 60 times. This impressive storage capacity makes the ocean a central player in the global climate system.
Carbon is mainly stored in the form of dissolved inorganic carbon (DIC), which consists of three components: dissolved CO₂, bicarbonate, and carbonate ions. These forms of carbon are in dynamic equilibrium, influenced by the pH of seawater.
Mechanisms of CO₂ Uptake from the Atmosphere
Currently, the oceans absorb about 25-30% of human-caused CO₂ emissions, equivalent to more than 2 petagrams (2 billion tons) of carbon per year. This uptake mainly occurs through the ocean’s surface layer, which varies regionally between 50 and several hundred meters thick.
The exchange of CO₂ between atmosphere and ocean is driven by the partial pressure difference between air and water. When atmospheric CO₂ levels are higher than in surface water, the ocean absorbs CO₂. Conversely, it releases CO₂ when concentrations in the water are higher.
The Physical Pump
The physical pump is especially efficient in regions where cold, salty water sinks, such as the North Atlantic and Southern Ocean. Dissolved CO₂ is transported to depth with the sinking water and remains stored there for decades to centuries. These regions are thus crucial for long-term carbon storage.
Recent research confirms that the Southern Ocean alone absorbs about 0.53 petagrams more carbon than it releases. This region is particularly important because cold Antarctic water rises and sinks again, enabling effective carbon transport to the deep ocean.
The Biological Pump
The biological pump works through marine photosynthesis. Phytoplankton, tiny marine plants, bind CO₂ from seawater and convert it into organic material. These organisms contribute about 50% to global photosynthetic carbon fixation.
Some of this organic material sinks as “marine snow” to deeper layers. These consist of dead organisms, feces, and other organic particles that sink under gravity. While much of the material is remineralized by microorganisms during sinking, a small portion reaches the sediments and is stored long-term.
The biological pump sequesters about 2.8 billion tons of carbon annually, keeping it out of the atmospheric cycle for at least 50 years. The value of this ecosystem service is estimated at $545 billion per year in international waters.
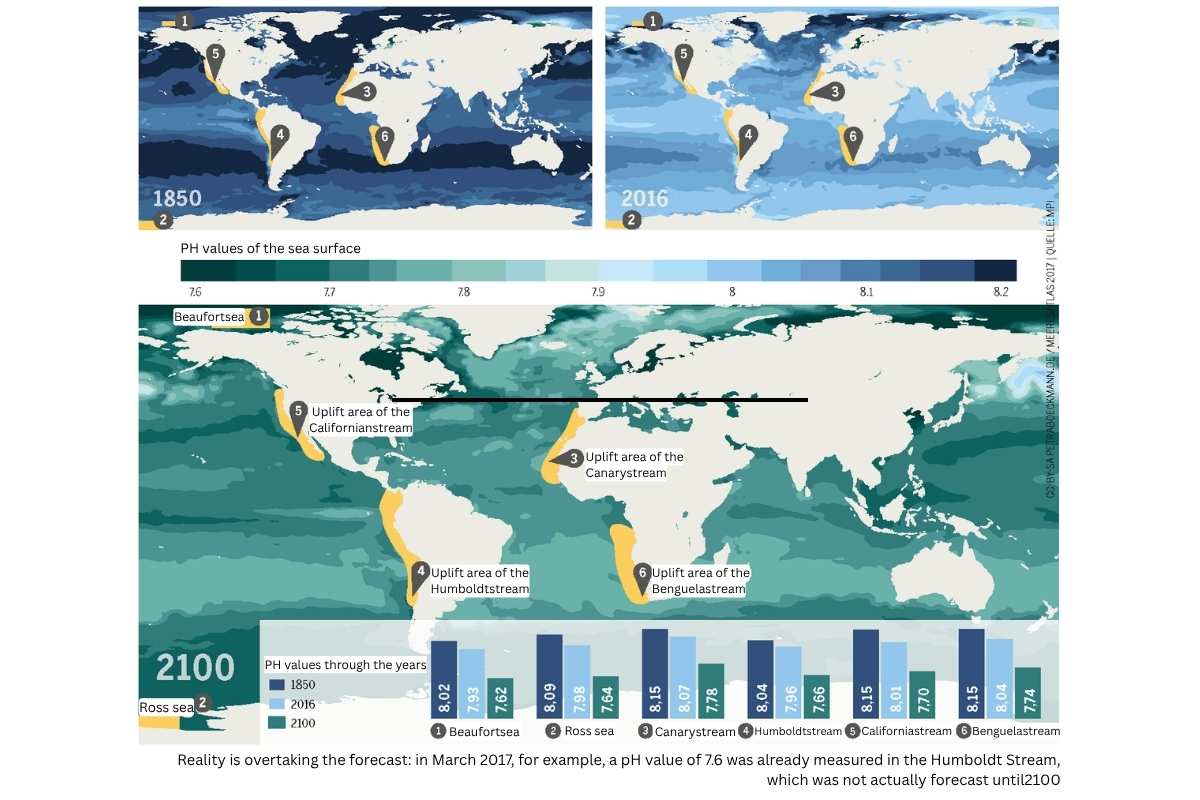
Regional Differences in CO₂ Uptake
The ocean’s ability to absorb CO₂ varies greatly between regions. In cold, stormy areas like the North Atlantic and Southern Ocean, uptake is especially high, benefiting from better CO₂ solubility in cold water and strong mixing processes.
Tropical upwelling areas, on the other hand, can act as CO₂ sources, as carbon-rich deep water reaches the surface and releases CO₂ to the atmosphere. These regional differences are crucial for understanding the global ocean carbon balance.
Recent studies also show that regional patterns can change. For example, in the subpolar North Atlantic, a weakening of CO₂ uptake is projected as deep water formation decreases.
Ocean Acidification as a Critical Consequence
The Acidification Process
CO₂ uptake leads to a series of chemical reactions in seawater, forming carbonic acid and lowering the pH. This process, known as ocean acidification, is one of the most severe consequences of CO₂ uptake.
Current measurements show that the global average surface ocean pH has dropped from 8.11 in 1985 to 8.04 in 2024, an 18% increase in acidity. Since preindustrial times, acidity has increased by 40%.
Impacts on Marine Organisms
Ocean acidification is especially problematic for calcifying organisms like corals, mussels, crustaceans, and certain plankton species. These organisms have difficulty forming shells and skeletons from calcium carbonate as seawater becomes more acidic.
Scientists use the aragonite saturation state as an indicator of the ability of organisms to form their calcareous structures. When the saturation state falls below 1, aragonite, a form of calcium carbonate, dissolves. Projections indicate that by 2100, about 61.5% of global ocean regions could fall below this critical threshold.
Rate of Change:
Current acidification is proceeding as rapidly as at any time in at least the last 20 million years. The World Meteorological Organization (WMO) reports that the oceans are acidifying 10 times faster than in the past 300 million years. This unprecedented rate of change makes adaptation difficult for marine organisms.

Limits and Future Developments of Ocean Uptake
Temperature-Related Limitations
As water temperatures rise, CO₂ solubility decreases, reducing the ocean’s uptake capacity. Warmer water can dissolve less CO₂, meaning that with ongoing warming, more CO₂ remains in the atmosphere.
Ocean Stratification as a Barrier:
Increasing stratification of the oceans due to warming is another limitation. As the surface layer warms, it becomes less dense and mixes less with deeper, colder layers. This slows CO₂ transport to the depths and reduces carbon storage efficiency.
Studies show that stratification in the upper 200 meters of the oceans increased by about 7% between 1960 and 2018. This development could significantly impair the oceans’ role as a CO₂ sink.
Projections for the 21st Century
Climate models predict that the ocean’s capacity to absorb CO₂ will decrease during the 21st century. In extreme warming scenarios, CO₂ uptake efficiency could peak by 2100 and drop to only half by 2300.
These projections are based on the formation of a surface layer with low alkalinity, which hinders CO₂ uptake. Extreme climate changes increase precipitation and slow ocean currents, leading to a warm freshwater layer on the surface that does not mix well with the more alkaline layers below.
New Developments and Research Approaches
Marine Carbon Dioxide Removal (mCDR)
Given the limits of natural CO₂ uptake, scientists are developing new technologies for marine carbon dioxide removal (mCDR). These ocean-based techniques aim to remove CO₂ from the atmosphere and store it long-term in the ocean.
In January 2025, NOAA published its first comprehensive plan for ocean carbon observation. This plan aims to improve the coordination and optimization of ocean carbon observation activities and to identify key scientific questions for the next 10 years.
Microbial Discoveries
New research has provided important insights into the role of microorganisms in the ocean carbon cycle. Scientists have identified a group of deep-sea microbes called “slow copiotrophs” that grow slowly but can efficiently break down hard-to-degrade organic carbon compounds.
This discovery offers new insights into carbon storage mechanisms and shows that even small changes in microbial communities can have major impacts on carbon storage.
Conclusion and Outlook
The ocean plays an indispensable role in the global carbon cycle and acts as an important buffer against the effects of human CO₂ emissions. With its enormous storage capacity of 38,000 gigatons of carbon and the ability to absorb 25-30% of anthropogenic emissions annually, it significantly slows the pace of climate change.
However, we face critical challenges. The ocean’s absorption capacity is not unlimited, and the resulting ocean acidification is a growing ecological problem. The unprecedented speed of acidification and ocean warming threaten marine ecosystems and could reduce the efficiency of natural carbon pumps.
Current research and developments in marine carbon dioxide removal offer hope for additional solutions. At the same time, new scientific findings on microbial processes and regional differences highlight the complexity of the ocean carbon system.
In the future, it will be crucial to both understand and protect the oceans’ natural capacities and to develop innovative technologies that can complement these natural processes. Only through a combination of drastic emission reductions and protection of marine ecosystems can we preserve the vital role of the oceans as climate regulators in the long term.
Author: Francesco del Orbe
Graphic: Heinrich Böll Stiftung / petraboellman.de / CC-BY 4.0